Introduction
Olutasidenib is a potent, selective, oral, small-molecule inhibitor of mutant isocitrate dehydrogenase 1 (mIDH1). Olutasidenib is approved in the US for the treatment of relapsed/refractory (R/R) acute myeloid leukemia (AML) based on the pivotal cohort (n=153) of a registrational Phase 1/2 trial (NCT02719574), which demonstrated a rate of complete remission (CR) or CR with partial hematologic recovery (CRh) of 35%, with a duration of response of 25.9 months. The Phase 1/2 study also enrolled patients with mIDH1 myelodysplastic syndromes/neoplasms (MDS), and here we report results from this subset of 22 patients.
Methods
The Phase 1/2 open-label study enrolled 332 adult patients with mIDH1 AML or intermediate-, high-, or very high-risk MDSfrom 57 centers in 9 countries. Inclusion criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 and adequate liver and renal function. Patients were R/R to prior therapy or, if treatment-naïve (TN), had to be eligible for azacitidine therapy (to be enrolled in a combination therapy cohort). Olutasidenib was administered at 150 mg BID (n=3) or 100-150 QD (n=3) as monotherapy or in combination with azacitidine (n=16), given at 75 mg/mm 2 daily for 7 consecutive days, in 28-day cycles. The primary efficacy endpoint for MDS was the CR rate, based on response criteria of the International Working Group in MDS. Secondary efficacy endpoints included overall response (CR/PR/marrow CR), time to response, duration of response and safety.
Results
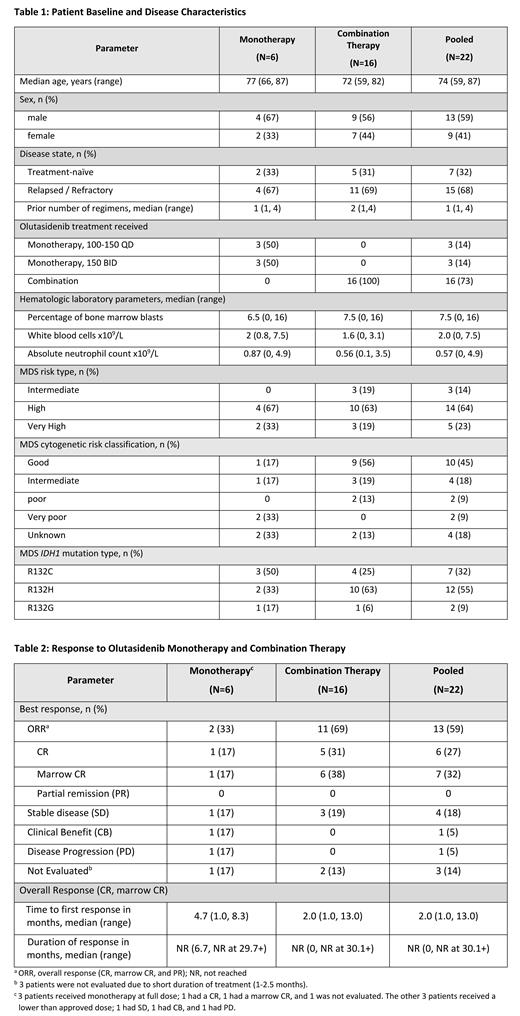
Twenty-two patients with MDS were enrolled, including 6 who received monotherapy (4 R/R and 2 TN) and 16 who received combination therapy (11 R/R and 5 TN). Baseline demographics and disease characteristics are shown in Table 1. The age ranged from 59-87 years, and 59% of the study population were male. Seven patients were treatment-naïve and 15 were R/R with 1-4 prior treatment regimens.
For the pooled Phase 1 and 2 data (n=22), 6 (27%) patients achieved CR and 7 (32%) patients achieved marrow CR with no PRs, generating a 59% overall response rate. The median time to response was 2.0 months. The median duration of response was not reached (NR) at 30.1+ months ( Table 2). The overall response rate was 2/6 (33%) for monotherapy and 11/16 (69%) for combination therapy. Three of 6 patients received monotherapy at a lower dose (100-150 QD) than the approved dose level, and none responded. Of the 3 patients who received monotherapy at the approved dose level (150mg BID), 2 (66%) responded. In the 7 TN patients, the overall response rate was 86%. In the 15 R/R patients, the overall response rate was 47%.
All patients with MDS experienced at least 1 treatment-emergent adverse event (TEAE). The most frequent TEAEs in the study were nausea, constipation, vomiting, thrombocytopenia, neutropenia, diarrhea, and fatigue. Grade 3 TEAEs occurred in 19/22 (86%) patients, and Grade 4 TEAEs in 9/22 (41%). The most frequent Grade 3/4 TEAEs reported were cytopenias. Grade 3 transaminitis occurred in 4 patients. Grade 3 differentiation syndrome was reported in 1 patient, who discontinued treatment after 23 days due to pneumonia, which lead to respiratory failure and death. TEAEs resulting in death occurred in 5 patients and included disease progression (3), pneumonia (1, mentioned above) and chest fungal infection (1). The overall safety profile in patients with MDS was consistent with what has been reported in patients with AML in this study (Watts, et al., Lancet Hematol, 2022; de Botton et al., Blood Adv, 2023).
Conclusions
Olutasidenib, both as monotherapy and in combination with azacitidine, induced durable remissions in patients with intermediate-, high-, or very high-risk MDS. Patients had varying treatment backgrounds, including treatment-naïve and up to four prior regimens. This treatment had a tolerable and manageable safety profile. These encouraging results, which warrant further investigation with a larger number of patients, show that olutasidenib has clinically meaningful activity in patients with mIDH1 MDS.
Disclosures
Cortes:Abbvie: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Research Funding; Gilead: Consultancy; Forma Therapuetic: Consultancy; Pfizer: Consultancy, Research Funding. Dinner:Rigel: Research Funding; Pfizer: Research Funding; Kite/Gilead: Research Funding; Novartis: Research Funding; BMS: Research Funding. Wang:Takeda: Consultancy; PharmaEssentia: Consultancy; Pfizer: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Kite: Consultancy, Speakers Bureau; Dava oncology: Speakers Bureau; Kura Oncology: Speakers Bureau; Jazz: Consultancy; GlaxoSmithKline: Consultancy; Gilead: Consultancy; BMS: Consultancy; Astellas: Consultancy, Speakers Bureau; Abbvie: Consultancy. Baer:Takeda (Inst): Research Funding; Kite, a Gilead company (Inst): Research Funding; FORMA Therapeutics (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; Abbvie (Inst): Research Funding; Kura Oncology (Inst): Research Funding. Watts:BMS: Consultancy; Rigel: Consultancy; Aptose: Consultancy; Takeda: Consultancy, Research Funding; Servier: Consultancy; Daiichi Sankyo: Consultancy; Reven Pharma: Consultancy; Rafael Pharma: Consultancy; Immune Systems Key: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal